The potential of placenta stem cells in medicine
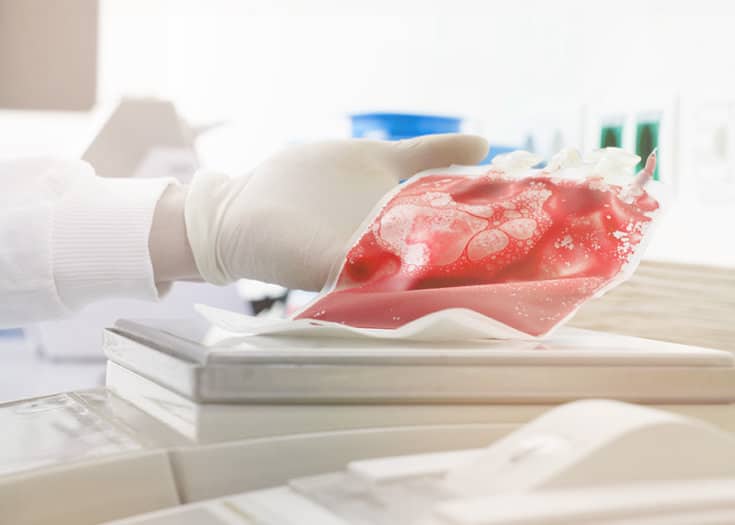
Several thousand umbilical cord blood storages have been carried out worldwide for many years. Umbilical cord blood contains precursor cells that are able to completely rebuild and restore the hematopoietic system from scratch in certain blood cancer treatments, e.g. after chemotherapy. In order to treat a child with Fanconi anemia (lack of red and white blood cells), allogeneic (transfer of cells, tissue or organs from one person to another) umbilical cord blood stem cells were first used medically for a similar application by the French doctor Eliane Gluckman in Paris in 1988. It is therefore both impressive and gratifying that, since the 1990s, such deep cold-stored umbilical cord blood units have already been used thousands of times (>>35,000, depending on the source) for sometimes life-saving cell therapy interventions. For medical reasons, the majority of these applications are covered by allergenically donated umbilical cord blood. However, treatment cases have also been described in which own or family stored umbilical cord blood was used therapeutically. This positive example should help to illustrate how such valuable biomedical resources from birth can be used therapeutically.
For autologous (transfer of the body’s own cells or tissues) applications, postnatal cells and/or tissues must be appropriately preserved and stored so that they can be made available for later applications. These efforts are aimed at aligning themselves as adequately as possible with the constantly developing field of cell therapy.
This rapid development in the preclinical and clinical research environment is evident from the fact that over 80,000 specialist articles are currently published in scientific journals every year and over 23,000 clinical studies on humans are already listed in international study registers (as at 2024, e.g. https://clinicaltrials.gov). The increasing number of applications for cell therapies in personalized and regenerative medicine is also reflected in the steady rise in the number of product approvals. In the past three years, the number of approved products has doubled, which currently results in over 40 officially approved cell therapy products in CH/EU/USA (as of 2024). It is also particularly noteworthy that a quarter of the product approvals are umbilical cord derivatives, which are currently used in cell transplants as part of routine applications.
The rapidly increasing number of clinical research results in humans proves that (stem) cells isolated from umbilical cord tissue or from the placenta can be used as starting material for even more diverse therapeutic treatments. These therapeutic effects arise either directly from the applied (stem) cells themselves, or indirectly by using certain factors that the cells release into their environment as soluble substances for therapeutic purposes.
Using (stem) cells isolated from the placenta or umbilical cord, more than 150 clinical studies on safety and efficacy (e.g. in the treatment of Crohn’s disease, diabetic neuropathies, foot diseases, etc.) are currently registered in international study registers. Cells and stem cells, isolated from umbilical cord tissue or placenta and stored at low temperatures (approx. -180°C) combine the therapeutically valuable properties that they:
- Can be isolated in application-relevant high quantities
- No additional surgical interventions are necessary for isolation and this poses no risk to the newborn, for example
- The isolated stem cells, stored in cold storage, can be used immediately and can also be used practically indefinitely (e.g. can be differentiated into various cell types such as nerve cells, insulin-producing beta cells, cartilage cells, etc.).
- The isolated cells are still relatively “young” and have been exposed to few environmental influences and can therefore also multiply well
Placental stem cells offer enormous potential for medicine, as they enable a wide range of therapeutic applications and promise significant advances in the treatment of diseases.
The greatest challenge when storing umbilical cord tissue is microbial growth. A natural birth is not sterile. For this not to affect the quality of the stem cell preparation later, Vita 34 tries to minimize the bacterial exposure already during transportation. The collection box is most suited for this purpose. It contains a special transport solution to place the umbilical cord in immediately after it was cut. When the umbilical cord arrives at the laboratory, it is again thoroughly disinfected, cleaned, and washed to make sure that even the last germ has been erased. Then, the umbilical cord sections are frozen at approximately minus 180 degrees Celsius in a special freezing bag, protected in an aluminum cassette..
By storing umbilical cord blood and umbilical cord tissue, you get the double stem cell power.

Compare products and prices
As a precaution, store either the umbilical cord blood or the umbilical cord tissue after the birth of your child. We offer both at different prices and terms.
Hello. Do you need further information?
Our guidebook for parents contains comprehensive information on the subject of stem cell storage. Order the guidebook by mail at no charge and without any obligation.